3-with-molecular-structure-and-versatile-applications.jpg)
When you see the term aluminum hydroxide formula, you might wonder, "What exactly is this substance, and why is it so important?" The answer is refreshingly straightforward: aluminum hydroxide is a common inorganic compound with the chemical formula Al(OH)3. This formula tells us that each molecule is made up of one aluminum atom (Al) and three hydroxide groups (OH).
So, what is the formula for aluminum hydroxide? It's simply Al(OH)3. You might also see it referred to as aluminium hydroxide formula in some contexts, especially outside the United States, or even as al al oh 3 in shorthand notation. The al oh 3 compound name is officially "aluminum hydroxide," but in scientific circles, it can also be called aluminic hydroxide or aluminum(III) hydroxide.
In nature, aluminum hydroxide appears most commonly as the mineral gibbsite, which forms in lateritic soils and bauxite deposits. Visually, you'll notice it as a white, amorphous powder—meaning it doesn’t have a defined crystal shape and looks somewhat like chalk or talc. If you’re curious about its texture or practical uses, imagine a fine powder that’s insoluble in water but surprisingly reactive in other scenarios.
This amphoteric nature is what makes aluminum hydroxide so versatile. It can act as either an acid or a base depending on its environment, which is key to its roles in medicine, industry, and environmental science. If you ever see the formula for aluminum hydroxide on a product label or in a chemistry textbook, you’ll now know exactly what it means—and why the al oh 3 compound name is so widely recognized in both science and industry.
For a deeper dive into its structure and properties, you can explore resources like BYJU'S Chemistry or Study.com, which offer additional context on this essential compound.
3.jpg)
When you look at the aluminum hydroxide formula—Al(OH)3—you might wonder what makes up this compound at the atomic level. To answer that, let’s start by breaking down the ions involved. The name of aluminum ion is simply “aluminum ion,” and it carries a +3 charge, written as Al3+. But how does it get this charge?
Imagine an aluminum atom. It naturally has three electrons in its outer shell. When it forms an ion, it loses those three electrons, becoming positively charged. This process creates the Al3+ cation. In the context of naming binary ionic compounds, this is a classic example: a metal (aluminum) forms a positive ion by losing electrons, setting the stage for ionic bonding.
On the flip side, the other key player is the hydroxide ion. The oh name chemistry refers to the “hydroxide ion,” and it’s a polyatomic ion made of one oxygen atom and one hydrogen atom. This group carries a -1 charge, written as OH−. Hydroxide is common in many bases and is known for its high reactivity.
So, how many hydroxide ions are bonded to each aluminum ion in Al(OH)3? The answer is three. That’s because the +3 charge of aluminum must be balanced by three -1 charges from hydroxide ions. This is a great example of the oh3 charge balancing principle in chemistry.
To create a stable, neutral compound, the positive and negative charges must cancel out. Here’s a simple visualization:
This charge balancing act is essential when naming binary ionic compounds and understanding what formula represents the compound formed from aluminum and hydroxide. The answer is Al(OH)3, which reflects the precise ratio needed for neutrality.
In summary, the aluminum hydroxide formula is a perfect showcase for the basics of ionic chemistry: a metal ion with a specific positive charge pairs with enough anions to achieve a neutral overall charge. This principle—the oh3 charge balance—applies to countless other compounds and is a cornerstone of chemical nomenclature and reactivity. For more on the ions involved in aluminum hydroxide, you can check resources like Study.com.
Now that you know how the formula comes together, let’s explore how these ions actually bond and arrange themselves in space—giving aluminum hydroxide its unique structure and properties.
When you first encounter the aluminum hydroxide formula, Al(OH)3, you might wonder: is this compound ionic, covalent, or a bit of both? Sounds complex? Let’s break it down in simple terms you can visualize.
Aluminum hydroxide is primarily an ionic compound. Here’s why: the aluminum ion (Al3+) is a metal cation, and each hydroxide (OH−) is a nonmetal anion. The bond between the aluminum and each hydroxide group forms because of the strong electrostatic attraction between these oppositely charged ions. In other words, the aluminum cation and hydroxide anion anion come together through ionic bonding. This is the classic scenario for aluminum hydroxide ionic or covalent discussions: the overall structure is held together by ionic forces, not shared electrons.
But there’s more. Within each hydroxide group, the oxygen and hydrogen atoms are connected by a covalent bond. This means the O-H bond is formed by sharing electrons, not by transferring them. So, you could say aluminum hydroxide has both ionic and covalent characteristics—ionic between Al3+ and OH−, covalent within each OH− group. This dual nature is why you’ll sometimes see the question aluminum hydroxide covalent or ionic come up in textbooks and exams.
If you’re a visual learner, the aluminium lewis structure is a great tool for understanding how the atoms and electrons are arranged. A Lewis structure shows all the valence electrons, bonds, and lone pairs, making it easier to see how the compound holds together.
Imagine the entire structure as a central Al3+ ion with three OH− ions arranged around it, each OH− acting as an anion anion. This arrangement ensures the overall neutrality of the molecule and highlights the unique mix of bond types.
Key Takeaway: Aluminum hydroxide is an ionic compound made up of a metal cation (Al3+) and three nonmetal anion anion (OH−), but inside each OH− group, the O and H are held together by a covalent bond. This duality is central to understanding its chemical behavior and properties.
This structure also explains why aluminum hydroxide is insoluble in water but reacts readily with acids and bases—the ionic bonds are strong, but the amphoteric nature allows the compound to interact with both types of reactants. As you move forward, you’ll see how this molecular arrangement underpins its amphoterism and solubility, which are explored in the next section.

When you read about the aluminum hydroxide formula, you might ask: what makes this compound so versatile in chemistry and industry? The answer lies in two key properties—its amphoteric character and its unique solubility profile. Let’s break these down so you can see why Al(OH)3 stands out from the crowd.
Imagine a substance that can “play both sides”—reacting with acids and bases, depending on what it encounters. That’s exactly what amphoterism means. Aluminum hydroxide is a textbook example of an amphoteric hydroxide, meaning it can act as either an acid or a base in chemical reactions. This dual behavior is central to its wide range of uses and is a popular topic in chemistry discussions about al oh 3 acid or base properties.
When aluminum hydroxide meets a strong acid like hydrochloric acid (HCl), it behaves as a base, neutralizing the acid and forming a salt and water. This is often referred to as the aluminium hydroxide + hcl reaction. Here’s how it looks in a simplified equation:
Al(OH)3 (s) + 3 HCl (aq) → AlCl3 (aq) + 3 H2O (l)
This reaction illustrates how Al(OH)3 can neutralize stomach acid, which is why it’s used as an antacid. The same principle applies to reactions with other strong acids, such as h2so4 (sulfuric acid), where aluminum sulfate and water are produced.
On the flip side, when Al(OH)3 encounters a strong base like sodium hydroxide (naoh), it acts as an acid, accepting hydroxide ions to form a soluble complex ion. This is the classic aluminium sodium hydroxide reaction:
Al(OH)3 (s) + NaOH (aq) → Na[Al(OH)4] (aq)
Here, sodium aluminate is formed, and the previously insoluble aluminum hydroxide dissolves. This transformation is a classic demonstration of amphoterism and is used in industrial processes to extract and purify aluminum compounds.
Now, let’s address a common question: is al oh 3 soluble in water? The answer is no—aluminum hydroxide is practically insoluble in water. If you try to stir it into a glass of water, you’ll notice it remains as a fine, suspended powder and quickly settles out. This property is crucial for its role as an antacid, as it allows the compound to neutralize stomach acid without being absorbed into the bloodstream.
Why does this matter? The low aluminium hydroxide solubility in water makes it ideal for applications where slow, controlled reaction is needed—like in pharmaceuticals or water purification. However, its ability to dissolve in strong acids or bases (but not in water) is a direct result of its amphoteric nature, which you’ll see referenced in both academic texts and industrial guides (see this resource).
Imagine you’re working in a lab and need to separate aluminum ions from a mixture. By adjusting the pH—adding acid or base—you can selectively dissolve or precipitate aluminum hydroxide. This is why understanding aluminium hydroxide + hcl and aluminium sodium hydroxide reactions is so valuable in both research and industry.
Key Takeaway: Aluminum hydroxide’s amphoteric nature means it can neutralize acids and dissolve in bases, but it remains insoluble in water. This unique combination underpins its roles in medicine, water treatment, and manufacturing.
As you move forward, you’ll see how these chemical behaviors translate into different crystalline forms and real-world uses—making the aluminum hydroxide formula a foundation for even more complex chemistry.
When you hear about the aluminum hydroxide formula, you might assume every sample of Al(OH)3 is exactly the same. But here’s a fascinating twist: while the chemical formula remains constant, the way the atoms are arranged in space can vary—leading to several distinct crystalline forms, or polymorphs. Sounds complex? Imagine building with the same set of blocks but stacking them in different patterns; you’ll get unique structures, each with its own properties. This concept, called polymorphism, is key to understanding why aluminum hydroxide appears in nature as different minerals with varied uses and characteristics.
Four major polymorphs of Al(OH)3 are recognized: Gibbsite, Bayerite, Nordstrandite, and Doyleite (reference). While their chemical makeup—one aluminum atom bonded to three hydroxide groups—remains unchanged, their atomic arrangements differ, resulting in unique minerals. This diversity is not just academic; it has real-world implications for mining, manufacturing, and the properties of end products like alumina hydrate and alumina trihydrate.
| Polymorph Name | Crystal Structure | Common Occurrence | Key Characteristics |
|---|---|---|---|
| Gibbsite | Monoclinic or Triclinic | Main phase in bauxite ore; widespread in lateritic soils | Soft mineral, easily cleaved; forms stacked sheets of linked octahedra; primary source of alumina trihydrate for industry |
| Bayerite | Monoclinic | Rare in nature; can form during Bayer process | Atomic layers stacked differently from gibbsite; sometimes called alpha-aluminum trihydrate |
| Nordstrandite | Triclinic | Rare; found in some bauxite deposits | Similar sheet structure to gibbsite but different stacking; less common in industrial applications |
| Doyleite | Triclinic | Extremely rare; discovered in Canada | Unique stacking of octahedral layers; primarily of interest to mineralogists |
If you’ve ever wondered what is alumina trihydrate or searched for the gibbsite formula, you’re looking at the most commercially important polymorph of aluminum hydroxide. Gibbsite, with its formula Al(OH)3, is the principal component of bauxite—the world’s primary source of aluminum. It forms soft, easily cleaved crystals made of stacked sheets, with each aluminum atom surrounded by six hydroxide groups in an octahedral arrangement (reference). This structure makes gibbsite a crucial source of hydrated alumina for refining into alumina hydrate and ultimately metallic aluminum.
You might ask, “Does the arrangement of atoms really make a difference?” Absolutely! The different stacking of layers in each polymorph affects how the mineral behaves—its hardness, solubility, and how easily it can be processed. For example, gibbsite’s sheet-like structure makes it softer and easier to refine, which is why it dominates industrial extraction. Meanwhile, bayerite and the rare forms nordstrandite and doyleite have properties that are more specialized or of interest mainly to geologists and researchers.
In ceramics and glazing, alumina hydrate (often derived from gibbsite) is prized for its high melting point, ability to suspend in slurries, and contribution to glaze opacity and matteness. Understanding which polymorph you’re working with can influence everything from product quality to process efficiency.
Key Takeaway: The aluminum hydroxide formula may be constant, but its natural and industrial forms—gibbsite, bayerite, nordstrandite, and doyleite—offer a spectrum of properties. This polymorphism underpins the versatility of alumina trihydrate in everything from aluminum production to advanced ceramics.
Next, we’ll see how these fundamental differences in form and structure translate into practical uses—especially in the medical field, where aluminum hydroxide’s amphoteric and insoluble nature make it a staple antacid and phosphate binder.
When you reach for an over-the-counter remedy for heartburn or acid indigestion, there’s a good chance the active ingredient is aluminum hydroxide antacid. But how does this familiar white powder actually work inside your body? Imagine you’ve just enjoyed a spicy meal and now feel that telltale burning sensation in your chest. That’s excess stomach acid, mainly hydrochloric acid (HCl), irritating your esophagus. Here’s where aluminum hydroxide medicine steps in.
For even better results, aluminum hydroxide is often combined with magnesium hydroxide—marketed as al mg hydroxide—to provide fast and balanced relief. Magnesium helps counteract one of the most common side effects of aluminium hydroxide: constipation. Together, these two ingredients balance each other out, making products like Maalox and Mylanta household names for digestive relief.
Beyond soothing stomach acid, aluminum hydroxide plays a vital role for people—and even pets—dealing with kidney disease. If the kidneys can’t filter phosphate efficiently, blood phosphate levels rise, leading to serious complications. Here’s where aluminum hydroxide medicine shines as a phosphate binder:
While this use is common in both human and veterinary medicine, it’s important to follow dosing instructions carefully. Overuse or high doses can lead to low blood phosphate levels or, rarely, aluminum toxicity—especially in patients (or pets) with compromised kidney function.
Key Takeaway: Aluminum hydroxide is a trusted remedy for heartburn and a valuable phosphate binder for kidney patients—human and animal alike. Use it as directed, be mindful of side effects, and consult a healthcare professional or veterinarian for long-term care.
Next, let’s see how the unique properties of aluminum hydroxide extend far beyond medicine, powering innovations in fire safety, water treatment, and more.

Ever wondered how everyday materials—from building panels to electrical cables—are made safer from fire? The answer often lies in the aluminum hydroxide formula, especially when used as aluminum hydroxide powder. When exposed to high temperatures, this compound undergoes endothermic decomposition, releasing water vapor and absorbing heat. This process cools the surrounding material and dilutes flammable gases, effectively suppressing the spread of flames. That’s why aluminum hydroxide is a staple additive in plastics, textiles, rubber, and coatings for construction, automotive, and electronics industries (Qingdao Pengfeng).
Because of these properties, aluminum hydroxide powder is the world’s most widely used flame retardant in terms of volume, often referred to as alumina trihydrate (ATH). Its effectiveness is so well recognized that it represents over 40% of all flame-retardant additives used in the polymer industry (ScienceDirect). For those working with or handling the compound, consulting the aluminum hydroxide sds (Safety Data Sheet) is essential for safe storage and processing.
But the story doesn’t end with fire safety. The aluminum hydroxide formula serves as a crucial intermediate in the production of alumina (Al2O3), also known as aluminum oxide. Through a process called calcination, aluminum hydroxide is heated to drive off water, leaving behind pure alumina. This material is a foundation for manufacturing everything from ceramics and abrasives to the aluminum metal used in countless industries. If you’re calculating the molecular weight of alumina for a specific process, understanding the transformation from Al(OH)3 to Al2O3 is fundamental.
For those managing bulk materials or working in manufacturing, referencing the aluminum hydroxide sds ensures compliance with regulatory standards and workplace safety protocols.
Imagine turning murky, contaminated water into crystal-clear drinking water. Aluminum hydroxide is a vital player here, too. When added to water, it acts as a flocculant: it binds with suspended particles and impurities, forming larger clumps (flocs) that can be easily removed. This process is essential in municipal water treatment plants and industrial wastewater systems, ensuring safe, clean water for communities and businesses.
Beyond heavy industry, you’ll also find aluminum hydroxide in cosmetics and personal care products. It acts as a gentle abrasive, a pigment, and an opacifier, giving creams and lotions a smooth, matte finish. In polymers, it serves as both a filler and a flame retardant, enhancing mechanical properties while improving fire resistance.
And while not a direct source of nh3 (ammonia), aluminum hydroxide’s role as a catalyst support and intermediate in chemical synthesis extends its influence to a variety of specialty and fine chemical processes.
Key Takeaway: The industrial reach of the aluminum hydroxide formula is vast—from fire safety and water purification to cosmetics and advanced manufacturing. Whether you’re handling aluminum hydroxide powder in a lab or referencing the aluminum hydroxide sds for workplace safety, understanding its properties and applications is essential for harnessing its full potential.
Next, let’s see how this versatile compound makes the leap from chemical precursor to high-performance aluminum metal, powering the industries of tomorrow.
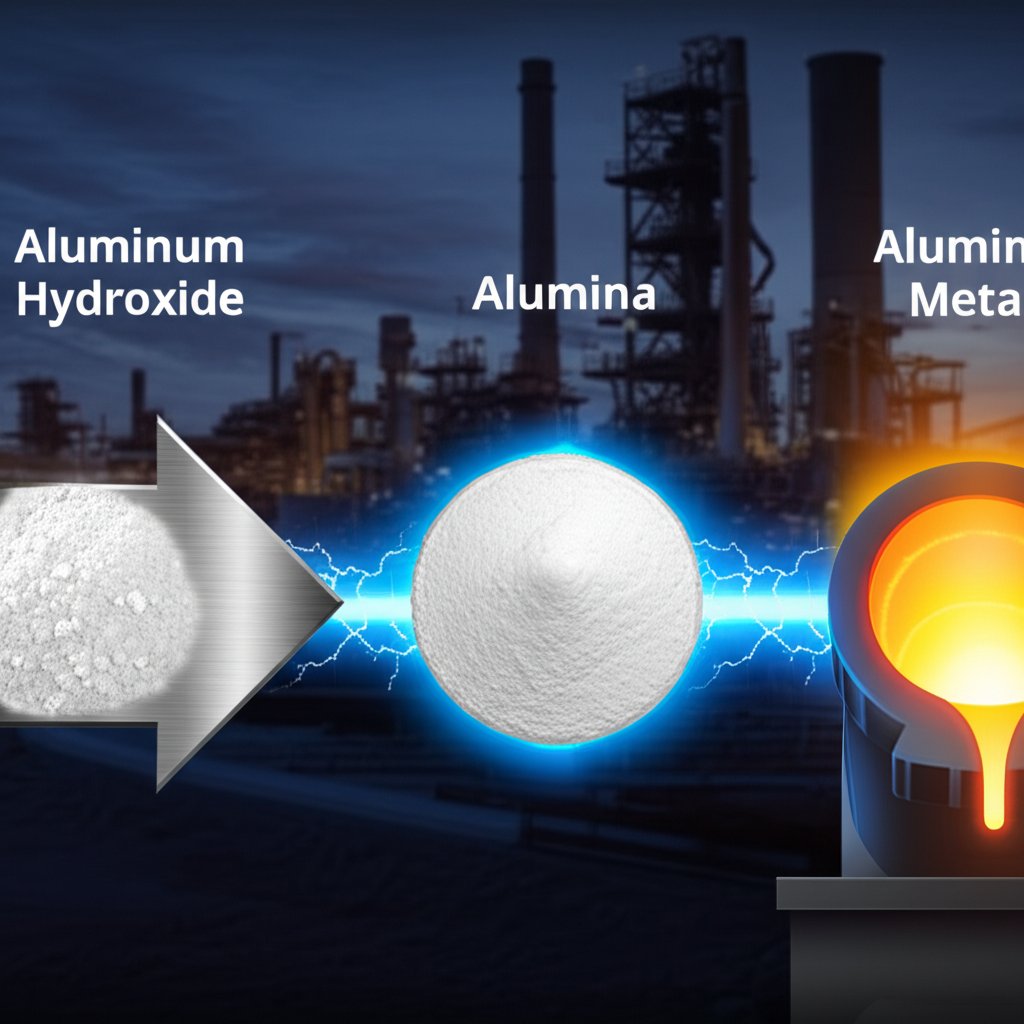
Ever wondered how a simple white powder like aluminum hydroxide is transformed into the lightweight, durable aluminum metal found in airplanes, cars, and skyscrapers? The answer lies in a fascinating industrial journey that starts with the aluminum hydroxide formula, Al(OH)3, and ends with high-performance aluminum products.
It all begins with the Bayer process, the world’s primary method for refining bauxite ore into aluminum oxide (alumina). Here’s how the transformation unfolds:
2 Al(OH)3 (s) → Al2O3 (s) + 3 H2O (g)
This reaction yields alumina (Al2O3), sometimes referred to as aluminum acid in older texts, though the term is not commonly used in modern chemistry.
Once alumina is obtained, it’s time for the final transformation. Through the Hall-Héroult process, alumina is dissolved in molten cryolite and subjected to electrolysis. This process separates pure aluminum metal from oxygen. The result? The shiny, lightweight metal that’s a cornerstone of modern engineering.
Imagine the precision required in aerospace, automotive, or architectural applications. Manufacturers depend on aluminum’s consistent quality, which can only be achieved through careful control of every step—from the initial aluminum hydroxide formula to the final metal profile.
For example, companies like Shengxin Aluminium in China leverage premium-grade aluminum to produce components that meet the strictest standards for strength, durability, and appearance. Their expertise in transforming raw alumina into high-quality aluminum profiles demonstrates how foundational chemical processes underpin the reliability of modern infrastructure and products.
Key Takeaway: The journey from Al(OH)3 to advanced aluminum metal is a testament to the importance of chemistry in industry. Understanding every detail—from al oh 3 molar mass to the complete aluminum hydroxide equation—ensures that the final metal is safe, non-toxic (addressing concerns like is aluminum oxide toxic), and perfectly suited for its intended use.
Whether you’re a student, engineer, or manufacturer, recognizing the connection between a simple compound like aluminum hydroxide and the high-tech world of aluminum metal is crucial for appreciating both the science and the industry behind everyday innovations.
The chemical formula for aluminum hydroxide is Al(OH)3, and its compound name is aluminum hydroxide. It consists of one aluminum ion and three hydroxide ions, forming a neutral amphoteric compound widely used in medicine and industry.
Aluminum hydroxide is practically insoluble in water. This property makes it suitable for use as an antacid, as it remains in the stomach to neutralize excess acid without being absorbed into the bloodstream.
Aluminum hydroxide neutralizes stomach acid by reacting with hydrochloric acid to form water and aluminum salts, providing relief from heartburn and indigestion. It also binds dietary phosphate in the gut, helping patients with kidney disease control phosphate levels.
Aluminum hydroxide is a key ingredient in fire retardants, a precursor for alumina and aluminum metal production, a flocculant in water purification, and is used in cosmetics and polymers for its flame-retardant and filler properties.
Aluminum hydroxide is first converted to alumina (Al2O3) through the Bayer process. The alumina is then smelted via electrolysis to produce aluminum metal, which is essential for manufacturing high-quality products such as those offered by Shengxin Aluminum.
 Servicio en línea
Servicio en línea 0086 136 3563 2360
0086 136 3563 2360 sales@sxalu.com
sales@sxalu.com +86 136 3563 2360
+86 136 3563 2360